The pipeline of new drugs coming to market continues to be steady, with 50 novel drugs approved by the FDA in 2021 and more on the way for 2022. The majority of these drugs are costly specialty medications, which make up nearly half of overall drug spending, even though their utilization is around 1%. As the number of approved drugs in 2022 rises, plan sponsors are eager to achieve significant savings through specialty drug management.
Diabetes is a growing concern in the U.S., with 35 million people living with the disease. GLP-1 is in the midst of an exciting new phase of development.


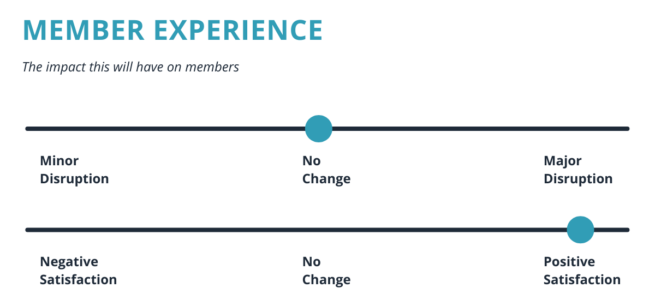
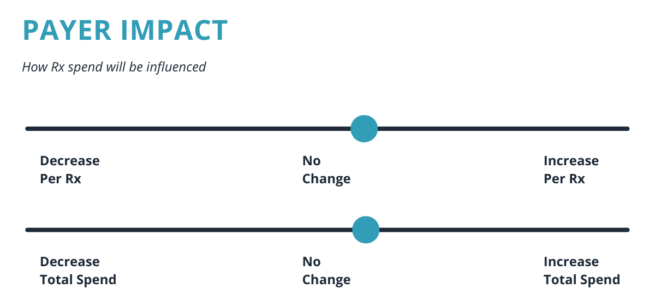
*Charts pulled from Elixer (https://blog.elixirsolutions.com/glp-1-agonists-pop)
For a more in-depth look at these recent advancements, click here.
Drug shortages have a significant impact on patient care and public health. Around 200 active drug shortages occur every quarter, including medications for COVID-19 and other drugs which were de-prioritized to allow vaccine production for COVID-19. The FDA continues to address the ongoing problem of drug shortages while maintaining and reviewing a list of extended use dates for specific lot numbers of drugs in short supply in the U.S. Here are the most recent drug shortages with information on the affected product, the reason for the shortage, available products, estimated resupply dates, and alternative drug therapy options.
The Centers for Medicare and Medicaid Services (CMS) have published the CY2023 Proposed Rule, the Advance Rate Announcement, and the CY2023 Medicare Parts C and D Annual Calendar via a Health Plan Management System (HPMS) memo, marking the milestones for this year and into 2023. Even though the proposed rule and rate announcement have not been finalized, we can begin preparing for 2023 based on published requirements effective 1/1/2023 and identify areas that may need adjustments when the proposal and rate announcement are finalized. CMS has indicated that plan sponsors should expect a greater level of Self-Audits with the launch of its Payment Recovery Information System (PRIS) Plan Portal. Furthermore, CMS began sending engagement letters to initiate routine audits in February and will continue through July, about a month earlier than in previous years. Although 2023 is months away, Healthgram is taking the necessary steps to prepare and assist our clients along the way.
Make sure your pharmacy benefits are on track by teaming up with a trusted partner. Healthgram’s pharmacy experts help our clients stay ahead in an ever-changing industry. For more on pharmacy trends and best practices, visit our insights page or contact our team directly.